With ResearchKit, the doctor is in… your iPhone
Apple’s open-source framework could literally be a life-changer, kick-starting a long-awaited revolution in medical research

March 9, 2015. Apple’s ‘Spring Forward’ event. ResearchKit is revealed, sandwiched between slots for HBO Now and the new MacBook, with the Apple Watch still to come. Apple’s wearable grabs most of the column inches, but ResearchKit was in many ways the most exciting announcement that day — and the most potentially transformative.
Apple describes ResearchKit as an open-source framework that enables everyone to do their part to advance medical research. Developers can craft apps with real-time tasks and surveys with customisable modules they can build upon and share.
People can easily opt into a study, secure in the knowledge data will only be shared with their permission. Moreover, they and researchers alike can reap hitherto unknown benefits. Unsurprisingly, the medical community is thrilled.
“I remember thinking the future just got much closer,” enthuses Rebar Interactive founder and researcher Rahlyn Gossen, who says although some of ResearchKit’s capabilities existed or had been envisioned elsewhere, they were now “integrated into a single, open-source platform” — something she never would have guessed we’d see in 2015, and certainly not from Apple.
Corey Bridges, CEO of Asthma Health developer LifeMap Solutions, believes healthcare was ripe for change: “I have a deep technological background and have had the good fortunate to be involved with several hugely disruptive technological plays. We’ve seen revolutions in news, music, and retail, and can now finally see something similar play out in healthcare. We’ll get rid of middlemen and better deal with patients.”
Dr. Ken Mandl, Harvard Professor at the Boston Children’s Hospital Computational Health Informatics Program, points to key benefits: moving research “outside the hallowed but very high institutional walls of academic medicine”; being a “platform for patient-powered science that resides in the pockets of millions”; and being a “toolkit for traditional researchers wanting to engage research subjects at home”.
In other words, medical research just got personal.
Breaking down barriers
The onus on individuals can be seen throughout ResearchKit. Ricky Bloomfield, MD, Assistant Professor, Internal Medicine-Pediatrics/Director of Mobile Technology Strategy at Duke Medicine, says everyone in the industry has “friends and loved ones with medical issues”, would “do anything to help them feel and be better”, yet often don’t know where to start.
ResearchKit removes barriers, allowing “anyone to participate in a study from the convenience of their own phone”. Dr. Mandl adds it dramatically simplifies consent and patient-reported responses: “Critical processes for engaging participants in research become easy and uniform — almost commodity.” Furthermore, Gossen says general public awareness of research remains low, which Apple’s marketing clout should improve.
None of these benefits should be underestimated. For example, Bloomfield says ResearchKit’s consent process breaks everything down into “easy-to-digest chunks with more explanation available as needed,” which you can tackle at your leisure, rather than “feeling pressured to consent by a study co-ordinator at your shoulder”. It does away with some selection biases by eradicating the need and time commitment required to visit — and often regularly return to — a specific institution in order to participate.
Instead of, as Bridges puts it, ending up with “only certain people who’ll jump through all the hoops,” you get scale. Sage Bionetworks Chief Commons Officer John Wilbanks says his organisation runs two of the five initial studies — Parkinson mPower, and Share The Journey (for breast cancer survivors) — and provides technical and governance support for the other three.
To date, ResearchKit has enabled the enrolment of “larger cohorts than traditional studies” and got data at “vastly deeper and more regular levels”.
Unlocking easy altruism
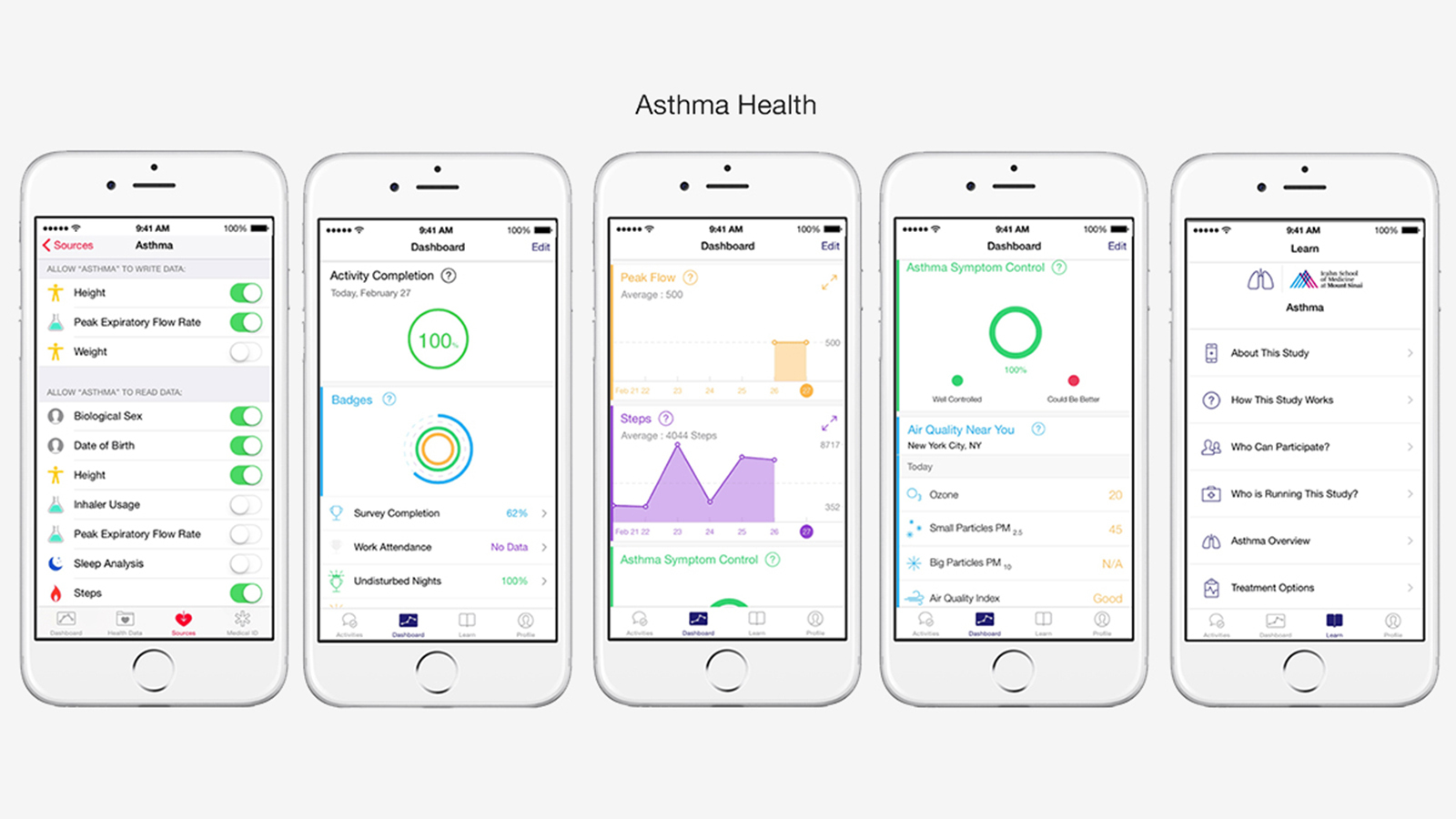
Without context, the term ‘larger’ alone may not impress, but Bridges states we’re talking “orders of magnitude greater” than participant numbers seen in traditional studies.
With the Asthma Health app, thousands signed up within a day. “Look at your average study prior to our ResearchKit app. A good one would have a few dozen or perhaps a hundred participants,” he continues. “But the ability to sign up and consent from your iPhone is glorious, and the scope of the investigations that can now be done — the granularity of data through having such larger populations — whether a specific or mainstream study… it’s just amazing.”
Mostly, Bridges thinks people do want to make the world a better place, but lack the time. Therefore, the ability for someone to join a medical study while sitting in their living room is a game-changer. “ResearchKit unlocks that ‘easy altruism’ people have, through being a low-friction way for someone to improve the world,” he says.
There’s also a cultural readiness (or at least acceptance) for this kind of system, in part realised by the increasing use of apps that monitor your wellbeing while simultaneously subtly gamifying aspects of your life. Bridges confirms LifeMap utilises its team’s expertise in user-retention, making use of best practice from experience in social media and gaming.
This has resulted in ‘sticky’ health apps that “could have taken the form of nagging you, but instead offer engagement and a sense of empowerment”.
Feedback loop
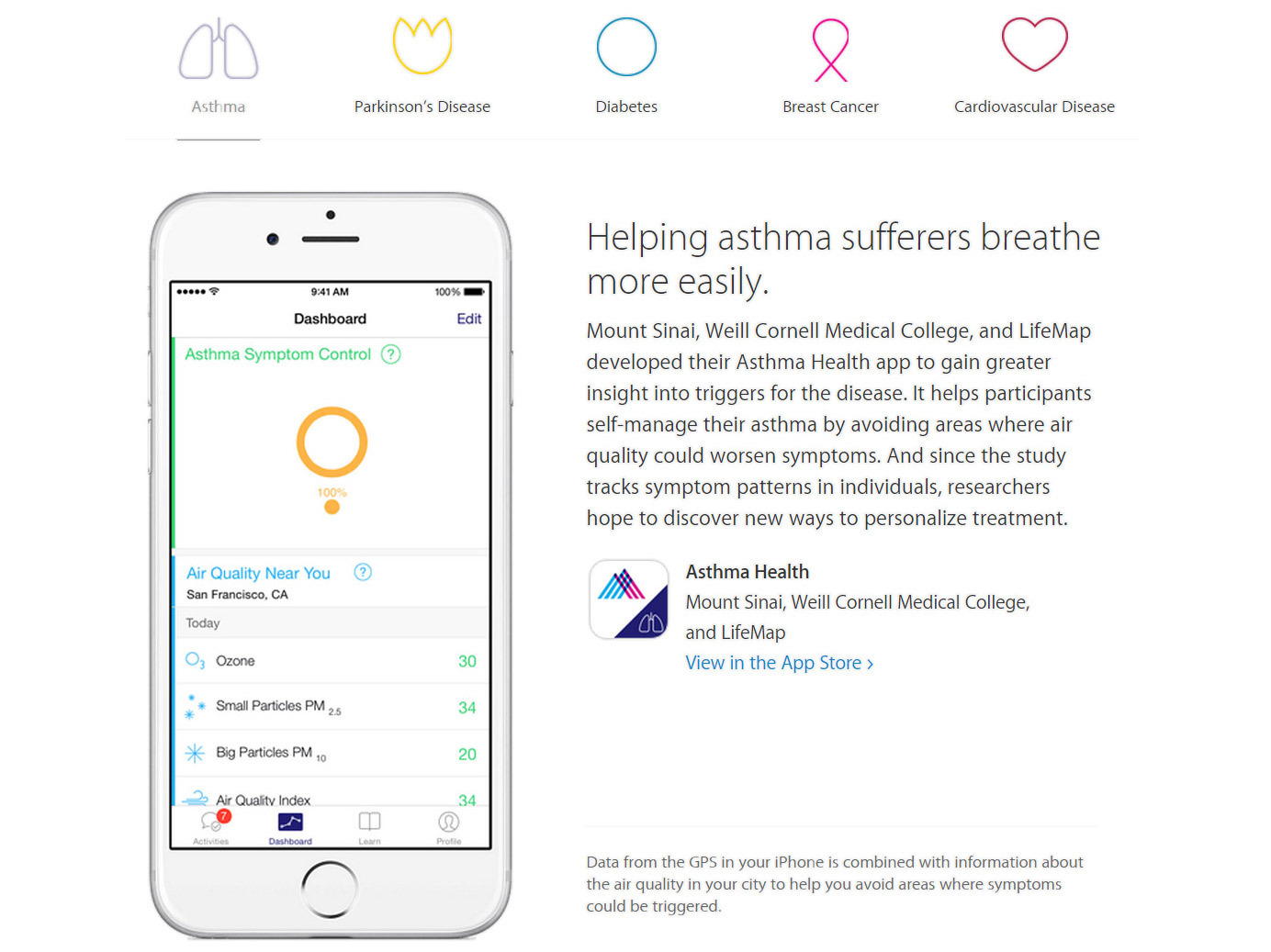
Another means of keeping everyone engaged with ResearchKit is through data. According to Dr. Eric Schadt, Director of the Icahn Institute at Mount Sinai, ResearchKit apps “enable seamless interactions between participants and app owners”.
The data collected can be acquired and processed, and then “meaningful feedback, advice or linking to healthcare professionals can be carried out to help the user better manage their condition”. By contrast, traditional large research studies have typically collected data on individuals, which was then processed and published, but any data meaningful to the participant rarely made its way back to them.
Wilbanks considers further positives: “The use of sensor data looks promising to track disease progression. This may well provide signposts for how to get closer to precision medicine. For individuals, the ability to affect the study itself through feedback will also be a breakthrough.”
By this, he not only means what makes someone feel better or worse, but also the study itself; Sage Bionetworks is already retooling protocols based on participant feedback: “People want things like a newsfeed, and to track other symptoms. That capacity is so much easier to attain when you can build the study around the person participating, not the clinical centre that hosts it”.
Read more › Jawbone UP3 review
Fine measurements
This sense of personalisation offers all kinds of potential. Dr. Schadt talks about the promise of coming up with “better, more accurate diagnoses”, due to the types of apps being built on ResearchKit enabling a “far better characterisation of your health than exists within the medical system”.
Additional data streams can further assist, for example taking into account an asthma patient’s exposure to potential triggers, through GPS tagging, weather data and pollen counts. “Because ultimately more data will be collected outside of the medical system than inside, someone will be able to better understand their condition and when best to seek help or obtain guidance on how to stay well,” he says.
Wilbanks adds that accuracy also removes an element of subjectivity that had been prevalent in conventional testing. “Physicians traditionally track each symptom in a disease by a given measure — so a person who has normal gait gets a certain numerical rating, and someone showing signs of unsteady gait gets another,” he explains. “But using mobile studies means each data-feed from remote sensors provides a multitude of features, and each of these can be gathered in real-time situations frequently.”
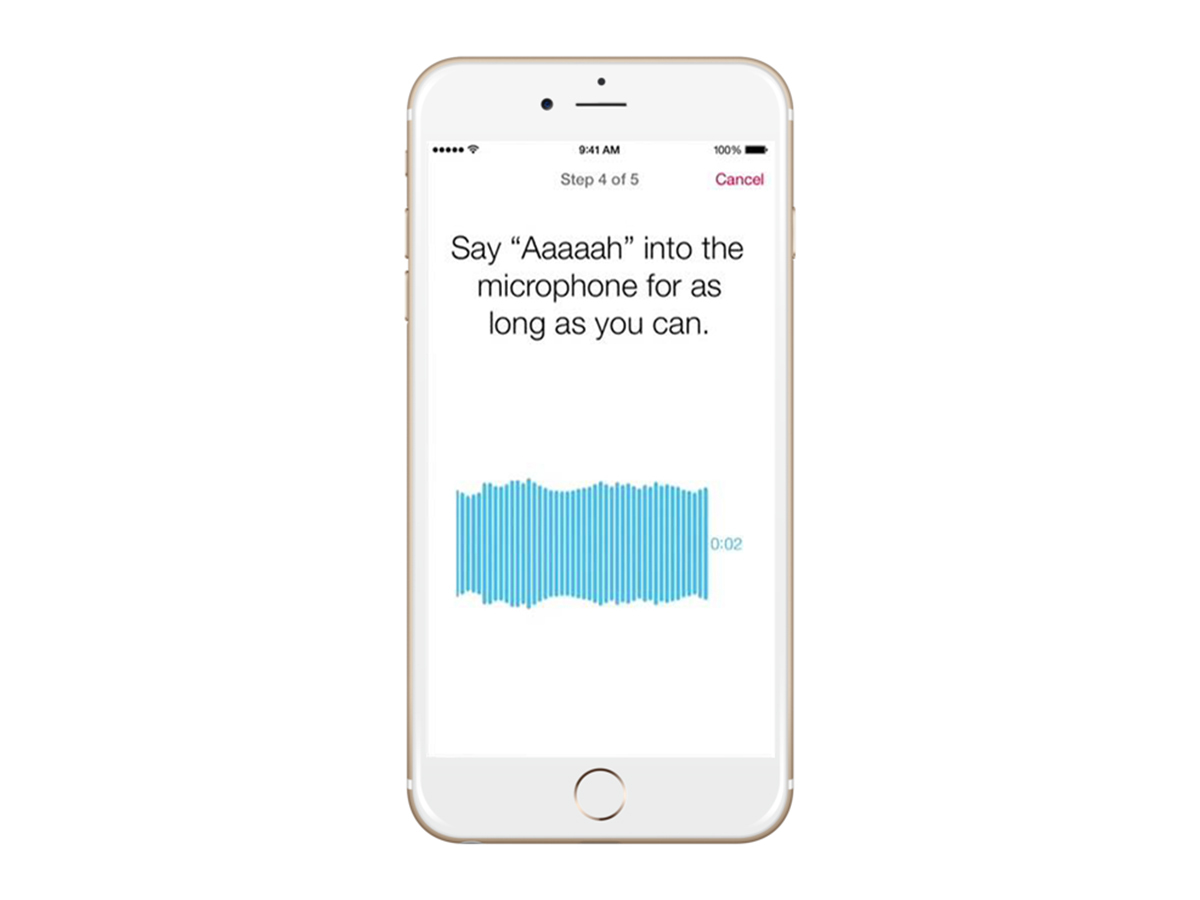
For the user, this data can be a useful means of tracking and monitoring. For the researcher, there are stricter limits, based on what the user permits, but Wilbanks isn’t concerned: “When measuring Parkinson’s disease, we might send a notification like ‘hold the phone and take 20 steps’, and only record the iPhone’s sensors for those 20 steps.
But those steps combined with other tasks, such as saying ‘Aaaah!’ into the microphone, tapping the screen, and completing surveys, gives us orders of magnitude more data on which to build classifiers of symptoms, of disease progression, and more.”
Keep it private

Regarding such personal data, there have been concerns about privacy, but Apple’s stance is clear: share data (if you choose to), but retain your privacy.
According to Bloomfield, Apple’s long demonstrated a commitment to privacy, now re-emphasised on entering the healthcare space; he adds: “Apple’s income primarily comes from hardware sales anyway, not monetising data,” which puts the company “in a unique position to guarantee your privacy”.
By contrast, Dr. Schadt explains traditional research studies are “basically ‘owned’ by the investigator, who decides who can see data and who can’t, rather than the contributor having that power”.
He dismisses concerns about people providing inaccurate information, to get into a study or even derail it: “It’s important to note you can provide misleading information to the clinical coordinator enrolling you in a traditional study, too.”
Although it’s perhaps easier to do this online than in person, Dr. Schadt says ResearchKit apps can weed out misleading information by asking questions in multiple different ways or flagging inconsistencies in answers, and take in objective information that does not depend on user input (such as health-tracking and local environmental data).
He adds you’d need to be very determined to mess around with a study anyway: “We’re seeing retention rates of over 60 per cent, weeks after launch. Those who trick the system to sign-up are unlikely to stick with a study over the long haul, given that there is an investment of time.”
A question of bias
With ResearchKit launching only on iPhone in the USA, there has been understandable unease regarding bias, with claims results may be skewed by the limited range of people who can take part. Bridges agrees that “clearly not everyone on the planet can enter a ResearchKit-enabled study”, but counters that traditional studies have been far more limited, in terms of geography, availability and accessibility.
More importantly, the two biggest issues will be dealt with in time: ResearchKit’s US-only nature is down to “regulatory hurdles”, and there’s nothing stopping the framework going beyond iPhone, since it’s open source.
In fact, Bridges appears surprised no-one’s yet adapted ResearchKit for Android, but then mulls it has only been in the wild for a few months: “And what’s happened so far has been pretty darn speedy — the rate of people knowing about it, taking action, and building on it.” He expects to see ResearchKit — or something similar — on Android soon, which will have to be open-source as well; interoperability, he reckons, will be inevitable.
Apple’s announcement ResearchKit would be open source got a cheer on March 9. It was an atypically Apple decision (if less so of late — programming language Swift subsequently followed suit), but essential. “It means someone can take the Parkinson’s work we’ve done and rapidly create an app for Huntington’s disease, without negotiations,” explains Wilbanks. “On another level, it increases the odds we get good, secure code, as the foundation for these apps.”
Moreover, he calls it a commitment to a “certain level of community”, where people aren’t suing over core functionality or ports to other systems — vital when it comes to furthering research.
Open up

Gossen adds open source is also a differentiator in a world where most available clinical research technologies are expensive closed solutions. “Research is relatively opaque to most people, in terms of technology and process.
Open up the technology and you open up the process. Open up the process, more people understand it, ultimately contribute and improve it. If ResearchKit can gain traction, it could make research much more transparent and democratic.”
She warns there are concerns with the type of studies ResearchKit is currently suited to — ‘minimal risk studies’, where the regulatory bar is low. But she nonetheless believes “regardless of whether ResearchKit ultimately gains mass adoption, Apple will speed innovation in research because it has chosen to enter the space”.
Industry insiders and outsiders will take note and “be encouraged to engage in activity that pushes the boundaries of the traditional clinical research model”. This may lead to major efficiency improvements in how research is conducted, resulting in rapid advances in medicine that will benefit everyone.
Read more › Withings Home review

Others foresee a similarly positive future. Wilbanks hopes ResearchKit is “part of a transformation to studies that are fully powered, that create reproducible results, that feed future research, and that put participants in their rightful place as full partners in the research enterprise — because the way we’ve been doing it doesn’t achieve any of those things very well”. And Bridges has no doubt this will all happen.
He speaks of lots of synergy already coming from ResearchKit, influencing best practice across mobile health. This will “accelerate a generation of new scientific insight and population insight,” potentially a virtue for all as the quantity and granularity of global and individual data increases.
This, says Bridges, combined with the increasing ubiquity of technology for gathering biometric data and a “cultural readiness — even eagerness” for new solutions, means ResearchKit will be a spearhead for a “radically different way for people to own and manage their own health through devices, sharing data where they will, under their own control”.
In only a few years, he believes we’ll “look back at the day ResearchKit was announced and say that was the inflection point when scientific medical knowledge — the learning thereof — accelerated”.